Researchers
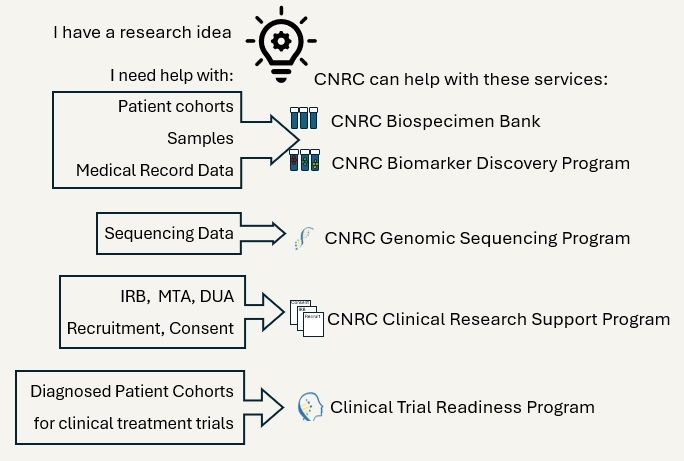
CNRC Biospecimen Bank
The CNRC has enrolled thousands of participants and established a comprehensive biorepository containing more then ten distinct specimen types. These samples are linked to both clinical and research data, providing a rich resource for scientific investigation.
CNRC follows standardized internal protocols to routinely collect biospecimens of broad interest, including DNA, serum, and plasma, from all participants. Additional specimen types can be collected upon request.
Samples currently available for collection: blood, DNA, RNA, serum, plasma, CSF, skin biopsy (including fibroblast cell line creation, muscle biopsy, and other tissue samples including autopsy specimens (e.g., brain).
- Clinical data: De-identified clinical data can be curated from patient medical records for research use.
Researchers can:
- Request existing samples and/or clinical data from patients with a specific diagnosis or other characteristics
- Develop prospective cohorts of specific patients including specialty sample and/or data type collection.
View available specimens in bank
CNRC Biomarker Discovery Program
This program provides relevant biospecimens for novel and existing biomarker studies of neurological disease. Researchers can utilize existing biospecimens or arrange for prospective collections tailored to the investigation including longitudinal collections, where necessary.
CNRC Clinical Research Support Program
This program provides an established Institutional Review Board (IRB) and research coordination infrastructure to supplement any study's regulatory needs and establish the appropriate agreements (e.g., IRB, MTA, and DTA, etc.).
CNRC Genomic Sequencing Program
This program can identify cohorts of individuals with neurological disease for DNA or RNA sequencing studies including exome, genome, transcriptome, or long-read sequencing and can assist with study design and providing data for subsequent analysis.
CNRC Clinical Trial Readiness Program
This program can assist with the identification of patient cohorts for current and future clinical trials including both natural history and treatment studies.
- Research assessments: Can administer common or specialty rating scales for various neurological diseases, on request