A UCLA-led research team has identified a genetic "brake" that prevents the body’s natural defense system from attacking solid tumors. By using CRISPR technology to delete a specific gene called FLI1, researchers were able to significantly enhance the ability of Natural Killer (NK) cells to survive and inhibit solid tumor growth.
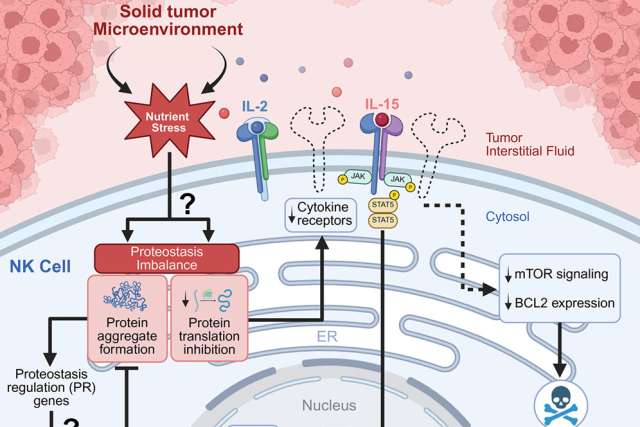
Natural Killer cells are the body's frontline defenders against cancer growth. However, advanced solid tumors are notoriously difficult environments for NK cells, while the cause has remained elusive. "This study identifies that the nutrients present inside the solid tumor are the critical factor that causes human NK cells to be ineffective," said senior author Dr. Timothy O’Sullivan, associate professor and Johanna F. and Joseph H. Shaper Family Chair of microbiology, immunology and molecular genetics at the David Geffen School of Medicine at UCLA and the study’s senior author.
The study, published in the peer reviewed journal Immunity, demonstrated that solid tumor-infiltrating NK cells rapidly accumulate aggregates of proteins, driven by the inability for NK cells to clear these protein aggregates when activated in the nutrients present in a solid tumor model. When these protein aggregates build up, NK cells lose their ability to receive activating signals and eventually die off, leaving the tumor to grow unchecked.
“Targeting pathways associated with metabolic stress will be important to reenergize NK cell responses against cancer. Understanding how NK cells respond to nutrients in their tissue environment is a major frontier of oncology research. Deleting the gene FLI1 in human NK cells allows them to survive better in solid tumors, delaying tumor growth and representing a promising strategy for a new immunotherapy,” said second author Wesley Armstrong, a graduate student in the UCLA medical scientist training program.
The study has some limitations. The tumor interstitial fluid medium (TIFM) used in the research came from a mouse pancreatic cancer model, so it may not perfectly match the nutrient environment in human pancreatic tumors or in other types of solid tumors. Because human blood and tumor fluid vary from person to person, TIFM is currently the most reliable and practical way to imitate the environment inside solid tumors in the lab.
Meanwhile, the team has submitted a provisional patent to edit FLI1 with CRISPR in human NK cells, said O’Sullivan, who is also a member of the UCLA Jonsson Comprehensive Cancer Center.
“Next-generation CRISPR targeting of FLI1 in patients, as opposed to engineering cells outside the human body, will be the next step in translating these findings to the clinic,” he said. “On the basic research side, we need to identify the precise combination of nutrients responsible for human NK cell inactivity, so we can find new ways to reengineer the metabolism of solid tumors to be more favorable for immunotherapy.”
Study co-authors are Jeong Hyun Ji, Joey Li, Areeba Lalani, Cassidy Lee, Varchas Bharadwaj, Kenneth Ho, and Jingtong Liang of UCLA, and Alexander Muir of the University of Chicago.
The National Institutes of Health (R01AI174519, R01AI186079, T32GM008042, 470 T32GM152342, T32AR071307, T32AI007323, F30AI181449, T32GM152342, 5P30AI028697), the UCLA Immunology Advisory Committee, a Ruth L. Kirschstein National Research Service Award (AI007323), the UCLA Molecular Biology Institute Whitcome Fellowship, a Brinson Foundation Junior Investigator Award and a Cancer Research Foundation Young Investigator Award supported this research.