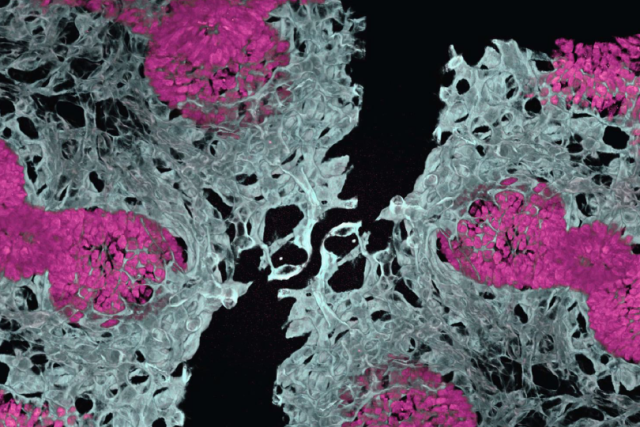
UCLA researchers have successfully grown miniature lungs from stem cells — complete with their own functioning blood vessel networks.
The groundbreaking work, published in Cell, marks the first time scientists have created lung organoids with integrated vascular systems that closely mirror how lungs develop in the human body.
The advance opens the door to potentially growing other vascularized organ models, including intestines and colons, providing unprecedented tools for studying diseases, testing drugs and developing personalized treatments.
"The earliest stages of human development are still a black box in many ways," said senior author Mingxia Gu, MD, PhD, a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. "This new method to vascularize lung organoids chips away at this black box by better recapitulating the natural organ development process."
The research team, which includes experts from Cincinnati Children's Hospital Medical Center, has already put the advanced organoid model to work, using it to uncover new insights into a rare and deadly congenital lung disorder that affects newborns.
Overcoming a critical bottleneck through unexpected discovery
Dr. Gu began this work at the start of the COVID-19 pandemic, when lung research was rapidly evolving and experiencing unprecedented attention.
Drawing on her background in pulmonary hypertension and vascular systems, she noticed a major gap in conventional lung organoid models: they lacked the blood vessel networks essential for lung function.
To solve this problem, the team initially tried the conventional assembly approach of growing organ components separately, using fluorescent markers to track different cell types, then combining them at a later stage. Red cells were supposed to become blood vessels, while green cells were meant to become lung tissue.
"We expected red-colored vascular networks and green-colored epithelium," explained Dr. Gu, who's also an associate professor of anesthesiology and perioperative medicine at UCLA. "But we actually found red-colored vascular networks and red-colored epithelium, meaning both cell types were surprisingly developing at the same time from the same starting material.”
This unexpected discovery led the team to abandon the assembly approach, which misses the critical early co-development window, and develop a strategy that more closely mimics how lungs actually form in the human body.
By allowing lung tissue and blood vessels to grow together from the beginning, the resulting mini-organs demonstrated greater cell type diversity, better three-dimensional structure, improved cell survival and more mature development compared to previous lab-grown organ models.
Tackling diseases that have historically been difficult to study
The team put the improved mini-lung models to immediate use, studying a congenital lung disorder called alveolar capillary dysplasia with misalignment of pulmonary veins, or ACDMPV. This devastating condition — characterized by severe respiratory issues — is caused by mutations in the FOXF1 gene and has no cure.
Previous attempts to study this disease have failed because the faulty gene primarily affects blood vessels and support cells, components that are missing from conventional organoid models.
Using the new method, scientists were able to take stem cells from patients with FOXF1 mutations and grow vascularized lung organoids that recreated both the primary blood vessel defects and the secondary lung tissue abnormalities that result.
“We wanted to show how this new organoid system provides researchers with a powerful new tool for understanding how blood vessels become specialized for different organs and how various cell types communicate with each other during both normal development and disease,” Dr. Gu said. “These vascularized lung organoids can be used to study any pulmonary vascular disease.”
Making mini-lungs even more lifelike
Even with built-in blood vessels, these lung organoids currently resemble fetal-stage human lungs. To make these mini-lungs more useful for medical research, the team is planning to introduce mechanical stretching and air exposure — mimicking the physical forces of breathing — to create the architecture found in more mature human lungs.
The team is also aiming to scale up production to generate large batches of these advanced organoids for drug development and testing.
“We’re essentially opening a window into cell-cell crosstalk during the early stages of human development, an area where our knowledge has been limited,” Dr. Gu said. “Scientists can now more effectively use human tissue models to study disease while reducing reliance on animal models to develop new medicines.”
Additional authors include: Yifei Miao, Nicole M. Pek, Cheng Tan, Cheng Jiang, Zhiyun Yu, Kentaro Iwasawa, Min Shi, Daniel O. Kechele, Nambirajan Sundaram, Victor Pastrana-Gomez, Debora I. Sinner, Xingchen Liu, Ko Chih Lin, Cheng-Lun Na, Keishi Kishimoto, Min-Chi Yang, Sushila Maharjan, Jason Tchieu, Jeffrey A. Whitsett, Yu Shrike Zhang, Kyle W. McCracken, Robbert J. Rottier, Darrell N. Kotton, Michael A. Helmrath, James M. Wells, Takanori Takebe, Aaron M. Zorn, Ya-Wen Chen and Minzhe Guo.
The research was supported by the National Institutes of Health, the Cincinnati Children’s Research Foundation, the American Heart Association, the Dr. Ralph and Marian Falk Medical Research Trust, the Brigham Research Institute and Erasmus MC.